1.Breating difficulty
2.skin sores, which may become a skin ulcer that heals very slowly
3.stuffy nose, runny nose, and nosebledds.
4.swallowing difficulty
TEST OVERVIEW for the Coronavirus(COVID-19)test
PERFORMANCE CHARACTERISTICS
The COVID-19 lgG/lgM Rapid Test (Whole Blood/Serum/Plasma) has been evaluated with the 126 samples obtained from patients exhibiting pneumonia or respiratory symptoms. The results were compared to Fluorescent Real Time Polymerase Chain Reaction (RT-PCR) or clinical diagnosis (including chest Computed Tomography and clinical signs etc.) of [Diagnosis and treatment of novel coronavirus pneumonia."
IgG/IgM Rapid Test results compared to SARS-CoV-2 RT-PCR results:
The sensitivity of lgM only test is 89.2% (100/112) and specificity is 100% (14/14).
RT-PCR Method testing was included as clinical site method. Nucleic acid was detected by probe fluorescence PCR. This product contained primers and probes (ORF1ab gene and N gene strains of coronavirus) and internal controls (housekeeping gene beta Globin gene sequences) used in RT-PCR for the in vitro qualitative detection of SARS-CoV-2 RNA in nasopharyngeal swab specimens. The novel coronavirus2019-ncov specific probe was respectively labeled with FAM fluorescence and ROX fluorescence, and the internal standard gene was labeled with VIC fluorescence. The minimum detection limit of the fluorescent RT-PCR assay is 10 copies/reaction.
LIMITATIONS
Coronavirus(COVID-19)test Rapid Test,Coronavirus Test Kit,Coronavirus Test,Covid-19 Test Dongguan Smart Furniture Co.,Ltd , https://www.smtfurniture.com
Lock Tradelink Xue Jian and Lewo General Manager Chen Qinghong signed a strategic agreement 
Lewo factory location 

Visit the Lewo factory 
Lai Zhongwu, Director Xue Jian, General Manager Chen Qinghong, General Manager Xiao Tiejun 

National free door-to-door installation commitment
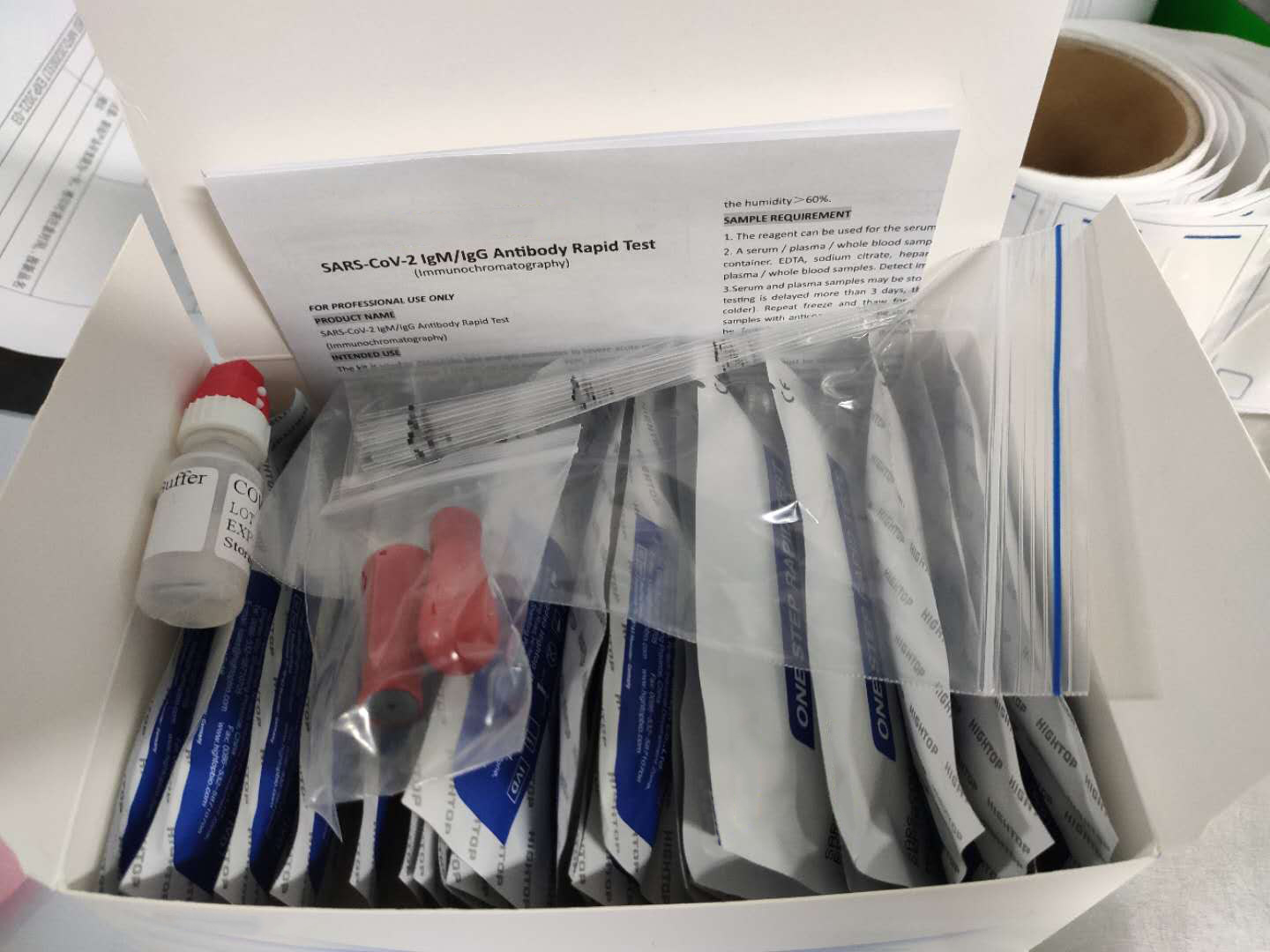
Description about Coronavirus(COVID-19)test Test
The Coronavirus(COVID-19)test is a solid phase immunochromatographic assay used in the rapid, qualitative and differential detection of IgG and IgM antibodies to the 2019 novel coronavirus in human whole blood, serum or plasma. The Coronavirus(COVID-19)test is intended for professionals within highly complex settings and or pharmacies. The test delivers rapid results between 2 and 10 minutes from individuals having signs and symptoms of SARS-CoV-2 infection
What conditions to do the Coronavirus(COVID-19)test?
COVID-19 RT-PCR Assay
Coronavirus(COVID-19)test
Positive
Negative
Total
Positive
103
9
112
Negative
0
14
14
Total
103
23
126
95% CI
Sensitivity
103/112
91.9%
(85.3% – 96.3%)
Specificity
14/14
100%
(76.8% – 100%)
Overall
117/126
92.8%
(86.9% – 96.7%)
The sensitivity of lgG & IgM test is 91.9% (103/112) and specificity is 100% (14/14).
"LAV0" Lewo joins the lock trade platform to improve the quality of after-sales service
Lock Tradelink mainly expands its national third-party installation service outsourcing platform with fingerprint lock as a typical smart home product. After nearly a year of hard work by Lock Tradelink team, APP and its operations have achieved nearly 10,000 service cases and become the industry leader. The nationwide network service system has been established, and it has been unanimously recognized by the industry and upstream manufacturers in terms of development concepts and development strategies, providing a strong driving force and foundation for the future development of the company.
Xue Jian, general manager of Lock Trade Co., believes that the installation and after-sales service of smart home products with fingerprint locks is the pain point and bottleneck of many manufacturers, and a large number of skilled installation masters have difficulties in connecting and working hard. Lock Tradelink will bring disruptive changes to the installation and after-sales service of locks, especially for online shopping. After the consumer has placed the lock order on the Internet, he can choose to install the locksmith master on the Internet through Lock Tradelink APP. Not only can the service price be transparent, real-time price comparison, but also the evaluation of the locksmith master installation service according to the past customers. The service comes from the main place to choose the locksmith master. After the locksmith master installs the service, the consumer can evaluate the service installed by the locksmith master, directly generate the master credit rating and star rating, and improve the service quality of the lock installation through the online real-time monitoring mode. Lock Trade is also very confident to do the best!
Lewo (Hongyang Metal) General Manager Chen Qinghong said that Lewo's fingerprint lock has been developed to the present, and has launched a series of high-quality products. The function and quality of the products have passed the test of the market and achieved a good reputation. The high-quality service Lewo fingerprint locks continue to develop and strengthen the foundation, enhance the brand's service quality is also the top priority of Lewo fingerprint lock future brand development plan. Lock Trade has many years of experience in installing after-sales service. Their strong implementation, service outlets all over the country, etc. are all needed for Lewo fingerprint locks, which is very compatible with the long-term planning established by our brand. We hope that through cooperation with Lock Trade, we can better provide consumers with high quality products and technical support.